As the reported COVID-19 death toll surpasses 1 million globally, all eyes are on the multibillion-dollar race for a vaccine. We sat down with Carter Gould, U.S. Biopharma Equity Analyst at Barclays, on Thursday, date, to discuss the state of COVID-19 vaccine trials and the major players racing toward the finish line.
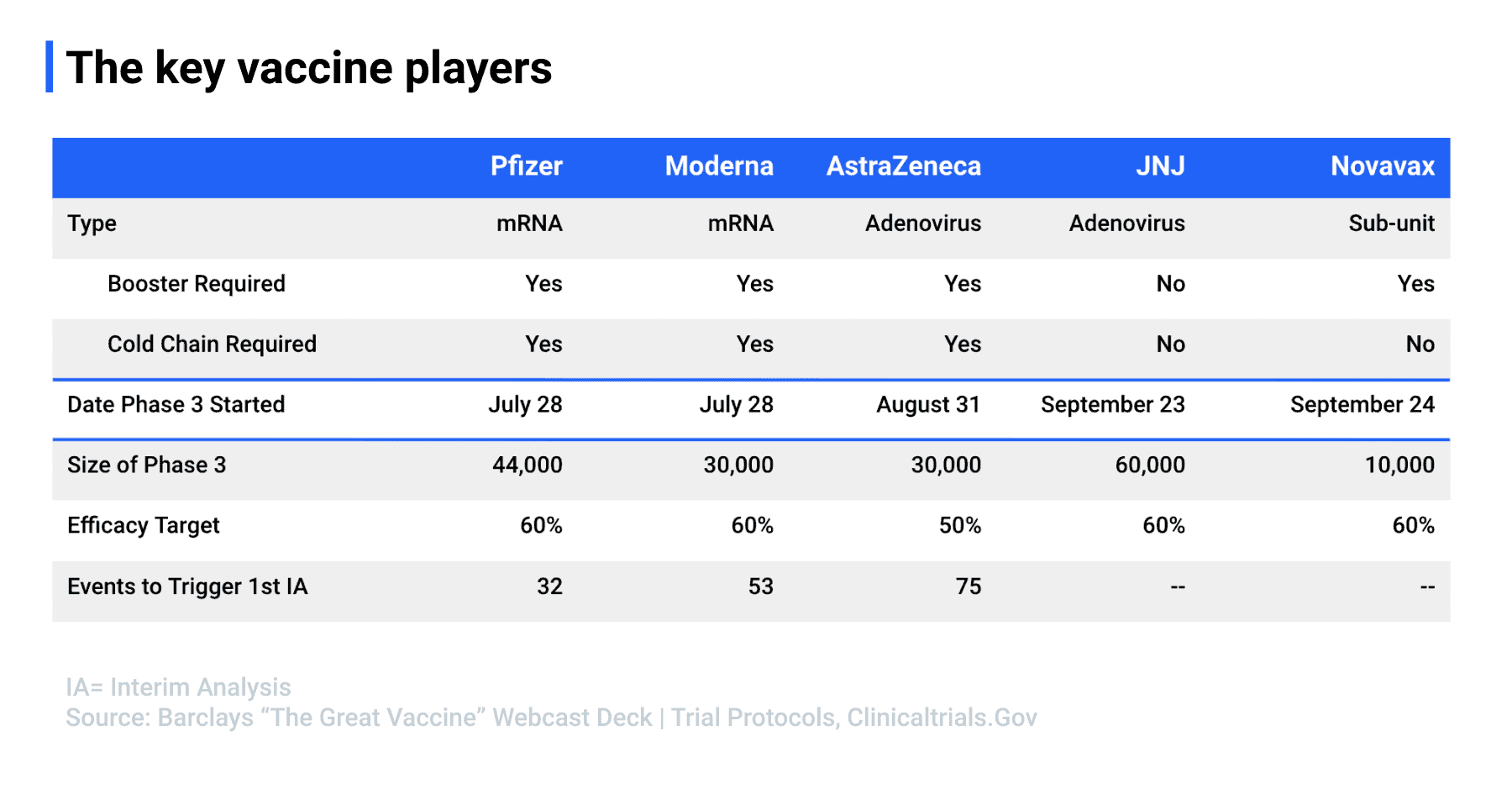
Source: Barclays “The Great Vaccine” Webcast Deck | Trial Protocols, clinicaltrials.gov
While the public waits, new FDA-approved plasma therapies have surfaced and Regeneron has created a promising antibody cocktail, which Gould summarizes in recent reports. Five major players (Pfizer, Moderna, AstraZeneca, Johnson & Johnson, and Novavax) have rapidly progressed in their vaccine trials, with some (Moderna and Pfizer) anticipating a faster launch, while others (AstraZeneca) have fallen behind due to adverse reactions.
While new therapies and promising Phase 3 trials provide hope, Gould acknowledges that it will take more than one company – or even two companies – to vaccinate enough of the population.
During our discussion, Gould also dove deeper into the most pressing questions from our clients including which of the five major players for the COVID-19 vaccine will likely be first, how manufacturing and distribution of vaccines should work and how many doses will likely be available.
To find out Carter Gould’s view on these questions and to read more of his research on the race to a vaccine and US Biopharma more broadly, login to AlphaSense or sign up for a free trial. To access the replay of the briefing, click here.